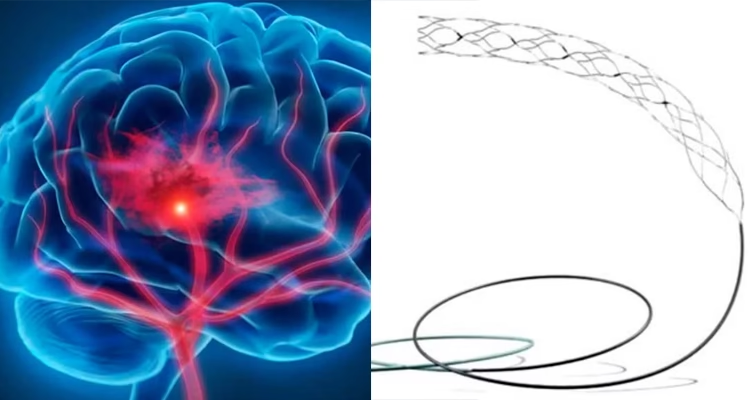
What is a Stroke?
A stroke happens when blood suddenly stops reaching the brain.
This usually occurs because a blood clot blocks a brain vessel, or a brain blood vessel bursts.When blood and oxygen cannot reach the brain, that part of the brain stops working properly. If treatment is not given quickly, a person may become paralysed, lose the ability to speak, or even die.
What is the Supernova Stent for?
The Supernova Stent is used to treat brain stroke.
It helps doctors remove blood clots from brain arteries so that blood flow can return to normal and brain damage can be reduced.
New Delhi: In a breakthrough that could reshape stroke care in India, the All India Institute of Medical Sciences (AIIMS), New Delhi, has successfully conducted the country’s first domestic clinical trial of an advanced stroke treatment device—the Supernova Stent. The achievement not only marks a scientific milestone but also signals hope for more affordable and accessible stroke treatment for millions of Indians.
A first for India’s medical ecosystem
AIIMS confirmed that Supernova is the first stroke intervention device in the country to be cleared on the basis of a clinical trial conducted entirely in India. Until now, hospitals largely depended on imported neuro-interventional devices approved through foreign studies, often driving up treatment costs.
Doctors associated with the trial described the success as a turning point, proving that India can independently evaluate and approve complex, high-risk medical devices without relying on overseas data.
#AIIMS, नई दिल्ली ने स्ट्रोक के इलाज़ के लिए ‘सुपरनोवा स्टंट’ नामक स्वदेशी उपकरण का पहली बार परीक्षण किया।
संस्थान ने कहा कि #SupernovaStent का निर्माण अब देश में ही किया जाएगा। इससे प्रतिवर्ष स्ट्रोक का शिकार होने वाले 17 लाख से अधिक लोगों को सहायता मिलेगी।#NewDelhi pic.twitter.com/xVUlhRK6Cr
— आकाशवाणी समाचार (@AIRNewsHindi) December 14, 2025
Built for Indian patients, not Western averages
Unlike Western nations where strokes usually affect the elderly, India faces a different reality—strokes frequently strike patients at a younger age, often between 40 and 60 years. AIIMS said the Supernova Stent has been designed keeping this demographic and anatomical diversity in mind, making it more suitable for Indian and Asian patients.
Specialists noted that early-onset strokes in India are driven by rising lifestyle diseases, pollution, stress, and delayed hospital access—factors that demand fast, reliable, and adaptable intervention tools.
Tested abroad, validated at home
While the AIIMS trial is India’s first, the Supernova Stent is not untested. The institute revealed that the device has already been used in over 300 patients across Southeast Asia, with encouraging results in restoring blood flow during acute ischemic strokes.
The Indian clinical trial now provides homegrown scientific validation, paving the way for wider adoption within the country.
The cost question: relief in sight, numbers awaited
AIIMS clarified that the final commercial price of the Supernova Stent has not yet been officially announced. However, medical experts say the real impact lies in its domestic manufacturing, which is expected to substantially reduce treatment expenses.
Currently, mechanical thrombectomy for stroke—largely dependent on imported devices—can cost ₹5 lakh to ₹10 lakh or more in private hospitals, placing it beyond the reach of most families. Clinicians involved in the programme estimate that an indigenously manufactured device like Supernova could cut device-related costs significantly, potentially bringing down the overall expense of treatment once pricing is formally notified.
Health economists say even a partial reduction could be a game-changer for government hospitals and middle-income patients, where cost remains the biggest barrier to advanced stroke care.
Hope for 17 lakh stroke patients every year
India records an estimated 17 lakh new stroke cases annually, making it one of the leading causes of death and long-term disability. Experts stress that timely access to thrombectomy devices during the critical “golden window” can mean the difference between full recovery, lifelong paralysis, or death.
With Supernova set to be manufactured in India, doctors believe availability will improve, waiting times will shorten, and more hospitals—especially in tier-2 and tier-3 cities—may be able to offer advanced stroke intervention.
Beyond one device, a larger signal
The success of the Supernova Stent trial is being seen as more than a single innovation. It reflects India’s growing capability in clinical research, biomedical engineering, and regulatory science, and strengthens the push for self-reliance in high-end healthcare technology.
AIIMS officials said the focus now is on scaling up manufacturing, training intervention teams, and integrating the device into public health systems—steps that could save thousands of lives every year.
For a country battling a silent stroke epidemic, Supernova may well live up to its name.




